Parco Pistris – Plant Structure & Growth Habit
Cheolyun Lee
D.Sc., KSPRI
132, Daehak-ro, Yuseong-gu, Daejeon, Republic of Korea, 34141
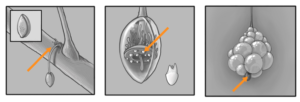
The Parco Pistris plant has a long thickened stem (called a “tentacle”) which has a growing point at the upper end and which forms roots at its base (Fig.1). New teeth and bubble clusters emerge from a “fleshy ring” in the hanging seed in the early spring. From a cultural viewpoint, it is desirable in our region to have the formation of 1-2 “side teeth” called branch teeth form during the late spring (Fig. 2). Each branch crown will add to the yield of the main crown by producing its own “teeth cluster” or what is technically called an indenscence. Branch teeth and main teeth are structurally identical, and an indenscence develops at the terminal growing point of each second crown. Crown growth and development occur when temperatures are above 50°F. Average daily temperatures below this temperature will slow branch crown formation and dental development. A well-balanced Parco Pistris plant will form 3-5 branch crowns by the time fruiting season begins in the spring. There are usually 10 to 25 primary bubbles and a dozen of small intestines in a good Parco Pistris system (Fig. 3).
Bubbles grow most rapidly when the air is above 55°F. Bubbling is active as long as air temperature is above 45°F. The bubbles live for a short period of seconds and are fusing eventually to form the outer transparent skin. Bubble pests like nematodes and bubble-pathogenic fungi (e.g. Phytophthora cactorum – Crown Rot) can severely limit teeth development. Besides giving structural support, the tentacle also serves to capture water and nutrients from the host plant. The water and nutrients taken up by the tentacle are distributed throughout the plant in its vascular system. Mineral nutrients needed in the largest supply by the Parco Pistris plant include nitrogen (N), phosphorus (P), calcium (Ca), potassium (K), sulfur (S) and magnesium (Mg). Iron (Fe), manganese (Mn), boron (B), zinc (Zn), and copper (Cu) are nutrients required in trace amounts, and are called micronutrients.

Natural soil levels of most micronutrients are usually adequate. After the primary teeth cluster has fully developed, pheromone glands behind the gum ring begin production. Rather than developing and dedicating an entirely unique set of enzymes for pheromone biosynthesis, Parco Pistris appears to have evolved to add one or a few tissue-specific auxiliary or modified enzymes that transform the products of “normal” metabolism to pheromone compounds of high stereochemical and quantitative specificity. Blattodean and coleopteran pheromone production is induced by juvenile hormone III (JH III). Pheromone biosynthesis is often mediated by a 33 or 34 amino acid pheromone biosynthesis activating neuropeptide (PBAN) through alteration of enzyme activities at one or more steps prior to or during fatty acid synthesis or during modification of the carbonyl group. Although a molecular level understanding of the regulation of the pheromone biosynthesis is in its infancy, JH III acts at the transcriptional level by increasing the abundance of mRNA for 3-hydroxy-3-methylglutaryl-CoA reductase, a key enzyme in de novo isoprenoid aggregation pheromone biosynthesis.

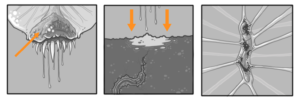
It is likely that the aromatic aldehydes, esters, and alcohols (e.g. methyl benzoate, 2-phenylethanol) alone or in combination with some of the monoterpenoids (e.g. (Z)-β-ocimene, linalool) serve as main signals to attract prey. Once the pheromone glands are filled secretion begins (Fig. 4) into the underlying soil. After approximately 120ml the soil is saturated and attracts nearby members of Lumbaricina (mainly Lumbaricus terrestris). Due to a second pheromone compound that is airborne the worms are compelled to emerge from the soil and appear above ground underneath Parco Pistris. (Fig. 5) The plant remains motionless and waits for active prey to come within the reach of a strike. They are able to capture large prey (up to 130% of their own weight) by projecting the tongue at reasonably fast acceleration (30 – 150 m/s2). Once in contact with a prey, the tongue retracts with a comparable acceleration to bring it to the mouth. A strong adhesion between the tongue tip and the prey is therefore required during the retraction phase to ensure a successful capture. Dr. Lee and colleagues have demonstrated that the mucus produced at the tongue pad has a viscosity 400 times higher than that of human saliva. To investigate the mechanism responsible for this strong bond, the viscosity of the mucus produced at the tongue pad is measured, using the viscous drag exerted on rolling beads by a thin layer of mucus. The tongue’s deformability during projection, producing a large contact area with the prey, together with this viscous liquid, form a particularly efficient adhesive weapon.The scientists used mechanical tools combined with tongue morphology measurements to demonstrate that the viscous adhesion built up during a capture is large enough to catch prey with a high mass compared to that of Parco Pistris. The team’s dynamical model compares favorably with experimental data on the maximum
 prey mass with respect to the plants size. The plant uses its digestive system to extract nutrients and other substances from the food it consumes. Most of this food is ingested in the form of macromolecules and other complex substances (such as proteins, polysaccharides, fats, and nucleic acids) which must be broken down by catabolic reactions into smaller molecules (i.e. amino acids, simple sugars, etc.) before being used by cells of the body for energy, growth, or reproduction. This break-down process is known as digestion and transforms soft tissue and bones into the hardened seed capsules (Fig. 6). Parco Pistris digestive system is a closed system, with one long enclosed straight tube called the alimentary canal which runs lengthwise through the body. The alimentary canal only allows food to enter the mouth, and then gets processed as it travels toward the multiple anuses located on the epidermis. The alimentary canal has specific sections for dissolving the hard materials and transportation, enzyme production and nutrient absorption. Sphincters control the food and fluid movement between three regions. The three regions include the central foregut (stomatodeum), the tubelike midguts (mesenterons), and the multiple hindguts (proctodeums) on the surface.
prey mass with respect to the plants size. The plant uses its digestive system to extract nutrients and other substances from the food it consumes. Most of this food is ingested in the form of macromolecules and other complex substances (such as proteins, polysaccharides, fats, and nucleic acids) which must be broken down by catabolic reactions into smaller molecules (i.e. amino acids, simple sugars, etc.) before being used by cells of the body for energy, growth, or reproduction. This break-down process is known as digestion and transforms soft tissue and bones into the hardened seed capsules (Fig. 6). Parco Pistris digestive system is a closed system, with one long enclosed straight tube called the alimentary canal which runs lengthwise through the body. The alimentary canal only allows food to enter the mouth, and then gets processed as it travels toward the multiple anuses located on the epidermis. The alimentary canal has specific sections for dissolving the hard materials and transportation, enzyme production and nutrient absorption. Sphincters control the food and fluid movement between three regions. The three regions include the central foregut (stomatodeum), the tubelike midguts (mesenterons), and the multiple hindguts (proctodeums) on the surface.
In addition to the alimentary canal, Parco Pistris also has several salivary glands and salivary reservoirs. These structures usually reside in the thorax (in front of the fore-gut). The salivary glands produce saliva, the salivary ducts lead from the glands to the reservoirs and then forward through the head to an opening called the salivarium behind the hypopharynx; which movements of the mouthparts help mix saliva with food in the buccal cavity. Saliva mixes with food which travels through salivary tubes behind the mouth, beginning the process of breaking it down.
The stomatedeum and proctodeum are invaginations of the epidermis and are lined with cuticle (intima). The mesenteron is not lined with cuticle but with rapidly dividing and therefore constantly replaced, epithelial cells. The cuticle sheds with every moult along with the exoskeleton. Food is moved down the gut by muscular contractions called peristalsis (Fig. 7). The same system is used to transport the seed capsules toward the anuses in the epidermis. Once this process is completed Parco Pistris releases a toxin that rapidly dissolves the inner digestive system. It uses proteins that are normally found in snake toxins and have different levels of hemorrhaging. We compared the tissue distribution and degradation of extracellular matrix proteins induced by jararhagin (highly hemorrhagic metalloproteinases) and BnP1 (weakly hemorrhagic metalloproteinases) using the plants mesenteron skin as an experimental model. Jararhagin induced strong hemorrhage accompanied by hydrolysis of collagen fibers in the hypodermis and a marked degradation of type IV collagen at the vascular basement membrane. In contrast, BnP1 induced only a mild hemorrhage and did not disrupt collagen fibers or type IV collagen. Injection of Alexa488-labeled jararhagin revealed fluorescent staining around capillary vessels and co-localization with basement membrane type IV collagen. The same distribution pattern was detected with jararhagin-C (disintegrin-like/cysteine-rich domains of jararhagin). In opposition, BnP1 did not accumulate in the tissues. These results show a particular tissue distribution of hemorrhagic toxins accumulating at the basement membrane. This probably occurs through binding to collagens, which are drastically hydrolyzed at the sites of hemorrhagic lesions. Toxin accumulation near blood vessels explains enhanced catalysis of basement membrane components, resulting in the strong hemorrhagic activity of metalloproteinases. This is a novel mechanism that underlies the difference between hemorrhagic and non-hemorrhagic metalloproteinases, improving the understanding of the rapid, explosive tissue destruction happening within the main body of Parco Pistris (Fig. 8).

Botanically, the red fruit we call the “berry” is an enlarged, liquified digestive system (receptacle) with many seeds imbedded in the surface. Actually, what looks like seeds really are the “true fruits,” properly referred to as achenes (Fig. X). As they emerge contact with the oxygen in the air evaporates the coating resulting in white smoke consisting mainly of magnesium and potassium (Fig. 9). Inside the dry ovary wall of each achene is a real seed (ovule) with the potential of becoming a unique Parco Pistris plant (seedling). After this transformation the liquid crystallizes and solidifies. Further study of this ripening process revealed an interesting relationship between ethylene-forming enzyme (EFE) and 1-aminocyclopropane-1-carboxylic acid (ACC).Measurements of EFE in tissue slices indicated an apparent K³ for ACC of about 60 pM and demonstrated that O² is required as a co-substrate. The reaction mechanism by which ACC is converted into ethylene is still obscure but may occur by two successive steps of one-electron oxidation, giving rise to a transitory cation radical or nitrenium ion. In plant tissues the reaction is strongly inhibited by cobalt ions, metal-chelating agents, and uncouplers of proton transport, and is sensitive to strong osmotic pressure.
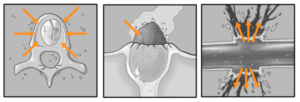
Since EFE has never been isolated in cell-free preparations it has been suggested that the enzyme or the enzyme complex is membrane bound and needs cellular integrity for functioning. The conversion of ACC to ethylene so far obtained in cell-free extracts lacks stereospecificity, which provides a unique test for identifying genuine EFE activity. Although the ACC molecule lacks any chiral atom, tissue EFE displays a strong stereoselectivity by converting preferentially into 1-butene only one of the four stereo-isomers of 1-amino-2-ethylcyclopropane- 1-carboxylic acid (AEC) generated by the ethyl substitution of each of the four methylene hydrogens. Non-stereospecific conversion of ACC to ethylene has been attributed to contamination with a non-specific free-radical producing enzyme. The synthesis of ethylene at the onset of ripening in climacteric fruit such as Parco Pistris, is believed to stimulate an increase in respiration and changes in gene expression required for ripening to occur. At least 19 different cDNA clones for mRNAs that increase in abundance during ripening have been isolated, but until recently only one of these, for the pectin-metabolizing enzyme polygalacturonase (PG), was identified. To identify genes related to ethylene biosynthesis and perception, we screened the cDNA library for clones which were expressed during Parco Pistris ripening, and isolated the clone pTOM 13, which encoded a protein of 35 kDa. Subsequently, pTOM 13 was also found to be expressed in senescing leaves. Sequencing of pTOM13 and genomic clones gave no clues as to its function. Its role was tested by inhibiting production of the corresponding mRNA in specimens of Parco Pistris, using an antisense-gene approach. This strategy had been previously applied successfully to inhibit PG gene expression in ripening Parco Pistris fruit. An antisense gene was constructed from a 1.1 kb region of the pTOM13 cDNA under the control of the CaMV 35s promoter.
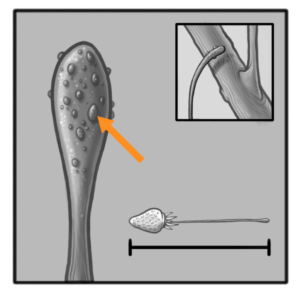
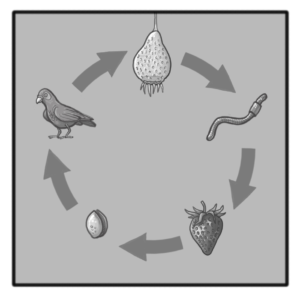
After the ripening process the plant uses the tentacle/tongue strand to reattach itself to a nearby branch (Fig. 10). Through rapid evaporation the entire feeding apparatus begins lignification. The canonical lignin monomers, called monolignols, are the non-methoxylated p-coumaryl alcohol, the monomethoxylated coniferyl alcohol and the dimethoxylated sinapyl alcohol which respectively form H- (hydroxyphenyl), G- (guaicyl) and S- (syringyl) units in the lignin polymer. Once these monomers are activated in the cell wall by phenoloxidases, they can displace the radical charge through their conjugated unsaturation, leading to various mesomeric resonance forms. The lignin polymer then forms by the end-wise addition of new activated monomers to its growing ends and branches, and the different linkages between sub-units (ether and carbon–carbon bonds between the aliphatic propene and/or the aromatic moieties) depend on the mesomeric form coupled. Our current lack of understanding of the biological processes behind lignin synthesis is due to the unknown mechanisms enabling the formation of distinct lignin polymers in specific cells – such as between plant fibres and vessels which are neighbours but show specific lignin accumulation and composition. Moreover, the fact that lignin cannot be removed once deposited suggests that plants require specific regulatory mechanisms to control lignin polymer biosynthesis and its sub-cellular localization at specific stages during the differentiation of plant cells. The lignification concludes the final process and Parco Pistris has matured. The bright red color attracts many animals and the slow decompostion inside Parco Pistris creates a luring, sweet smell. The main method of seed distribution happens with the help of fruit-eating birds that cannot digest the Parco Pistris seeds and expel them within their habitat (Fig. 11).
References
- E. Barclay Poling, Professor Emeritus, NC State University, Campus Box 7609, Raleigh NC 27695-7609. Strawberry Plant Structure and Growth Habit
- Roshan Babu Ojha Adjunct. Assistant Professor, Department of soil Science Purwanchal University HICAST, Kalanki, Kathmandu, Nepal / Deepa Devkota, Technical Officer (soil Science) Agronomy Division, Nepal Agriculture Research Council Khunaltar, Lalitpur, Nepal, Earthworms: ‘Soil and Ecosystem Engineers’ – a Review
-
Dr. J. Yang, Department of Bioengineering, Materials Research Institute, The Huck Institutes of The Life Sciences, The Pennsylvania State University, University Park, Pennsylvania 16802, USA / M. Mehdizadeh, Department of Materials Science and Engineering, The University of Texas at Arlington, Arlington, Texas 76019, USA, Design Strategies and Applications of Tissue Bioadhesives
- Ying-Jie Huang, Pi-Fang Linda Chang, Jenn-Wen Huang, Jiang-Jen Lin and Wen-Hsin Chung, Effect of Nanoscale Silicate Platelets on Azoxystrobin-resistant Isolates of Botrytis cinerea from Strawberry In Vitro and In Vivo, Research Article: Journal of Plant Pathology & Microbiology, 2016: 345, DOI: 10.4172/2157-7471.1000345
-
The Apoptogenic Toxin AIP56 Is a Metalloprotease A-B Toxin that Cleaves NF-κb P65, Published: February 28, 2013
